This study aimed to investigate the effects of END on cardio-respiratory capacity and nasal function, considering different facial types.
MethodSixty-three healthy adolescents participated in a randomized protocol in two conditions: with experimental END and with placebo. Maximal oxygen uptake (VO2max) was estimated and rating of perceived effort (RPE) after cardio-respiratory testing and peak nasal inspiratory flow (PNIF) were evaluated. To assess the differences between the use of the END and placebo regarding physiological assessment measures and between the PNIF, RPE, and VO2max measurements with each of the groups studied, the Student's t-test for paired samples was used. To investigate the existence of a statistical difference in the PNIF and VO2max measurements. and RPE when participants used experimental END between the three facial types, Analysis of Variance (ANOVA) with one factor (OneWay) was used. The Wilcoxon test was performed for comparisons between two periods in relation to a variable of interest. All results were considered significant at the 5 % significance level (p < 0.05).
ResultsThe results demonstrated that the use of END provided significant improvements in VO2max, an increase in PNIF and a reduction in RPE. Participants with a dolicofacial facial profile had a higher VO2max than the braquifacial and mesofacial profiles.
ConclusionWe conclude that END is an effective tool to improve cardio-respiratory capacity, reduce RPE and optimize nasal function in adolescent futsal players.
The effectiveness of the external nasal dilator (END) has been recognized in improving respiratory capacity and nasal function in adolescent athletes.1–4 Macfarlane and Fong4 examined the effectiveness of END in a randomized manner in adolescent athletes and observed a significant increase of 2.9 % in aerobic performance. They also reported an improvement in the subjective sensation of exertion, compared to placebo. Dinardi et al.1 observed a significant improvement in aerobic capacity and nasal function in healthy adolescents and those with allergic rhinitis when using END, compared to placebo. Furthermore, a significant reduction in nasal resistance was observed in both groups, as assessed by rhinomanometry. The hypothesis was raised that END may contribute to increasing minute ventilation (MV), partial pressure of oxygen in the alveoli, in addition to providing better respiratory perception during exercise and a reduction in the perception of dyspnea (ventilatory effort). Such mechanisms favor an increase in the amount of oxygen available to the respiratory muscles, allowing the athlete to perform better due to greater efficiency in energy production during exercise.5,6 However, all these studies mentioned did not evaluate the effectiveness of the END according to the facial type of the adolescents. Griffin et al.7 used the END and observed a significant reduction in perceived exertion, heart rate, ventilation, and oxygen consumption during submaximal exercise in adults, compared to placebo. They reported that ethnicity may have significantly influenced these results. Facial anatomy, characterized by variations in bone and muscle structures, as well as different face types, braquifacial (short and wide), dolicofacial (long and narrow), and mesofacial (proportional between vertical and horizontal diameters), can influence respiratory capacity and impact the effectiveness of the END. Recently, a systematic review with meta-analysis evaluated the effectiveness of the END in improving performance during aerobic exercise and observed that there was no significant difference between the use and non-use of the device.8 The variables analyzed included maximum oxygen uptake, heart rate, and rating of perceived effort (RPE). Despite these results and the consolidated understanding of the mechanism of the END, which involves increasing the area of the nasal valve and, consequently, airflow, there is still a gap in the literature regarding the application of the device in different types of exercises, sports modalities, analysis methodologies and facial shapes.
To date, no studies have been identified that explore the effects of the END during exercise in relation to different facial types. Thus, the objective of this study was to investigate the effects of the external nasal dilator, considering facial types, on the cardio-respiratory capacity and nasal function of adolescent futsal players.
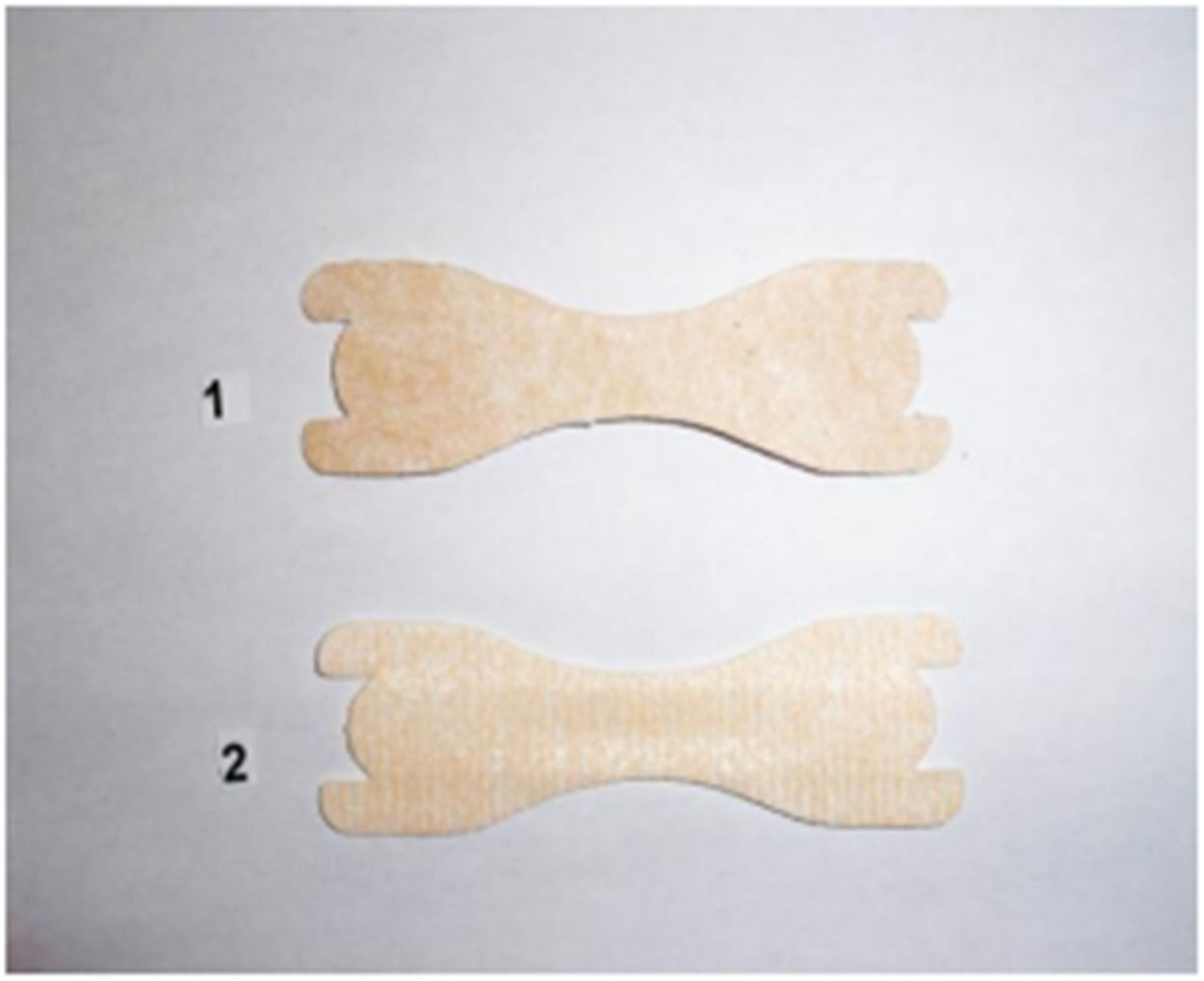
MethodsSixty-three healthy, randomly selected adolescents of both sexes volunteered for this study [32 males and 31 females aged 15 to 17 years (mean age: 16.0 ± 0.8 years; height: 1.74 ± 0.11 cm; body mass: 59.7 ± 8.5 kg)], who practiced futsal three times a week and each training session lasted one hour and thirty minutes. Adolescents with negative responses to the questions about asthma and allergic rhinitis in the International Study on Asthma and Allergies in Childhood (ISAAC) questionnaire9 were included. Individuals with any chronic disease detected by anamnesis, such as moderate to severe adenoid hypertrophy, verification of facial features and oral breathing posture, high-arched palate, crossbite, bacterial sinusitis diagnosed clinically by detection of purulent nasal secretion, postnasal drip, pain on facial percussion associated or not with headache and fever, deviated nasal septum, nasal polyps and active upper airway infection were excluded.The inability to perform the appropriate maneuver to obtain peak nasal inspiratory flow (PNIF), the inability to adapt to the END or the absence of the informed consent form signed by the adolescent and/or the parents or guardian. Likewise, those who voluntarily withdrew from the study and who did not return at the second moment to perform the tests were excluded. The risks and benefits of participation were provided in writing, and informed consent was obtained prior to data collection. This study received ethical approval from the necessary institution prior to commencement and conformed to the Code of Ethics of the World Medical Association (Declaration of Helsinki). The END used in the study is the one found on the market in Brazil (ClearPassage®, RJ, Brazil), available in three sizes: small, medium and large, which may be used by children, adolescents and adults. The sizes chosen were small and medium, according to each participant’s need. Application of the END was carried out according to the manufacturer’s instructions and it was inserted by one of the researchers. Participants were advised not to touch the device, which should be placed where they cannot see it. It functions in a simple, painless and non-invasive manner. Each strip has two parallel plastic bars that gently open the nostrils. The placebo END (Fig. 1) was made from an adhesive plastic tape without the acrylic strip responsible for dilating the nostrils. The devices were similar in appearance (size, color and shape), particularly at the extremities.
This study used a randomized, double-blind, crossover design. All individuals randomly participated in two situations, one using the Clear Passage® END and the other using the placebo nasal dilator. The nasal dorsum of each participant was cleaned with cotton moistened with alcohol before fixing it to the ENDs of the nostrils. Participants were instructed not to touch the device.
Cardio-respiratory testing and rating of perceived effort (RPE)To assess cardio-respiratory capacity, the Léger running test was performed, also known as the 20 m shuttle run aerobic test, on a court or in a suitable space.10 This test assessed the maximum aerobic capacity of the participants, in which a free area measuring 20 m in length was required, delimited between two parallel lines. At the evaluator's signal, the participants began the route running together (maximum 10), at a pace marked by a specific audible “beep” for the test. In the first stage the speed was 8.5 km/h, with an increase of 0.5 km/h in each of the following stages. Each stage lasted approximately one minute. Beeps were emitted at specific intervals for each stage. At each beep, the participant should have one foot crossing one of the two parallel lines, that is, leaving one of the lines running towards the other and crossing it with at least one foot when hearing a beep and returning in the opposite direction. The exclusion zone (limit) of the test was two meters before the parallel lines: any participant who was before this zone was warned by a beep to speed up the run. If the participant could no longer keep up with the pace, the test was interrupted and ended when he or she could no longer keep up with the rhythmic pace. The duration depended on the cardio-respiratory fitness of each participant. The objective of the test was to estimate VO2max, the intensity of which increases progressively throughout the evaluation, which lasted a maximum of 21 min. The Borg scale was used to describe the individuals' perception of physical effort.11 RPE was measured immediately after the cardio-respiratory test.
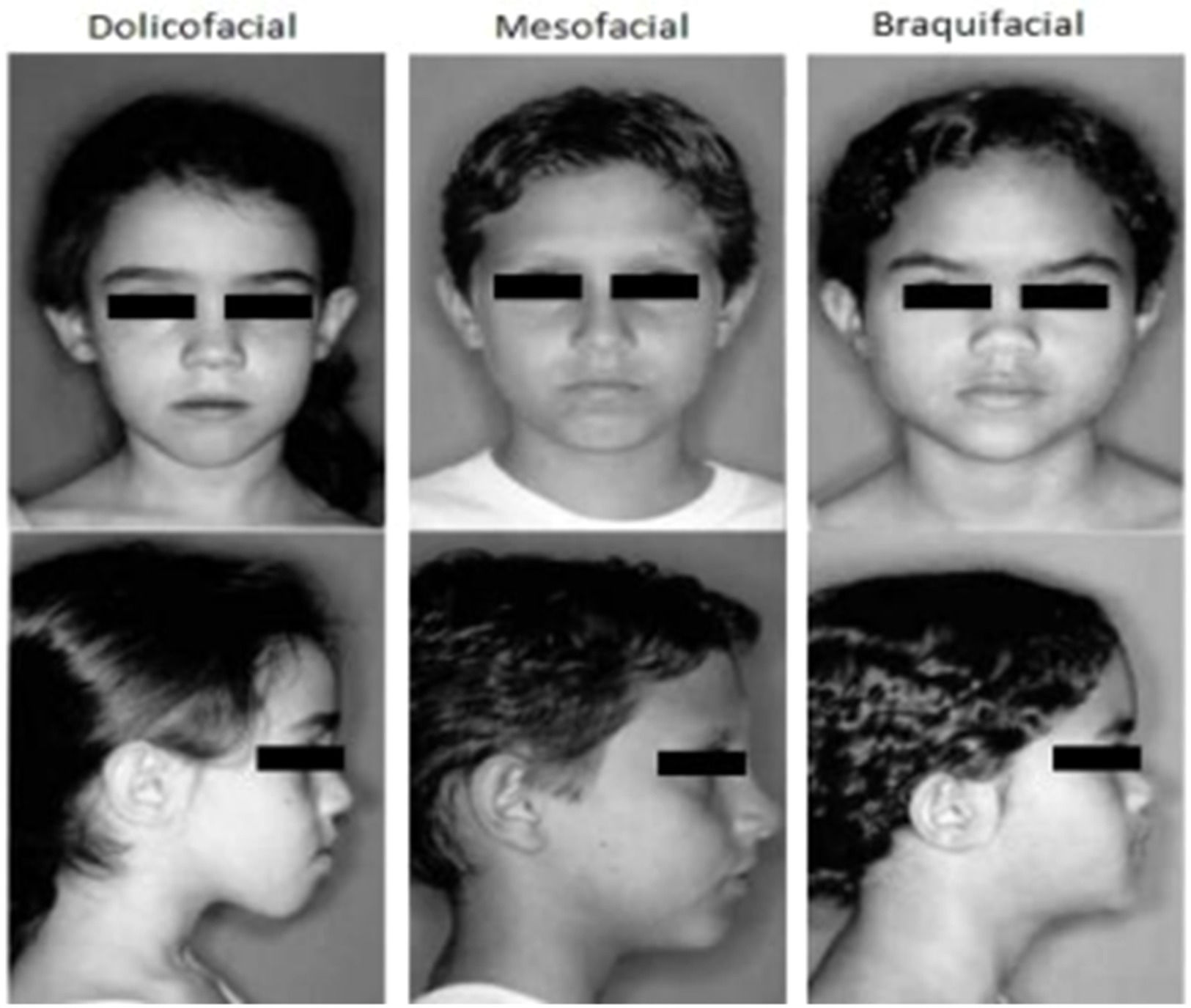
Assessment of facial typologyThe participants' facial type was classified into three categories: Dolicofacial, Mesofacial, and Braquifacial (Fig. 2).12 The dolicofacial type has a vertical growth direction greater than the horizontal, a longer face with a long, oval head. The braquifacial type has a horizontal growth direction greater than the vertical, a shorter face with a square, wide head, and, finally, the mesofacial type exhibits proportional growth between the vertical and horizontal diameters. To determine the facial type, the guidelines of Godinho et al.12 were followed. The participant remained seated in a chair, with the head erect, and the classification was performed using the facial ectoscopy method (observation) by two independent observers.
Facial profiles12.
Before checking the PNIF, the participant performed the usual nasal hygiene, lightly blowing their nostrils. Standing, the face mask was carefully adapted, instructing him to take vigorous nasal inspiration with his mouth closed from the residual volume until he reached total lung capacity. The equipment used was the in-check-inspiratory flow meter (Clement Clarke, Harlow, England). Three measurements were taken and the one with the highest value was chosen.
Data collection and analysis planThe assessments were carried out at two different times: the first involved health status, collection of anthropometric measurements, obtaining the PNIF and assessment of the facial profile, and the second time involved cardio-respiratory testing and assessment of RPE. After consulting the randomization (participants randomly allocated), the END (experimental or placebo) was applied, three PNIF measurements were obtained, the cardio-respiratory test was applied and RPE was assessed immediately after the test performed by independent examiners. In the second time, 48 h later and at the same time as the previous assessment, the participants who used the experimental END in the first assessment used the placebo END, and vice-versa. The PNIF was obtained with the experimental or placebo END before the cardio-respiratory test. The evaluator who placed the END and the participant were unaware of whether the END used in the first or second time was the experimental or placebo END. The prescription and assessment of the tests were performed by another independent observer.
Statistical analysisFrequency, mean, and standard deviation (SD) were calculated to describe the sample. To assess the differences between the use of the END and placebo regarding physiological assessment measures and between the PNIF, RPE, and VO2max measurements with each of the groups studied, the Student's t-test for paired samples was used. The normality of the distribution of differences between the two measures evaluated was verified using the Shapiro–Wilk test. To verify whether the sample was satisfactory for comparison between the measurements with and without the END, the effect size was considered based on Cohen's “d”, calculated with the aim of obtaining the standardized magnitude of the differences between two observed measurements/factors of interest. Cohen developed a “d” assessment scheme, with d = 0.20 meaning a small effect, d = 0.50 an intermediate effect, and d = 0.80 a large effect. The Kappa coefficient was used to describe the agreement of one or more evaluators in the assessment of the type of face.13 To investigate the existence of a statistical difference in the PNIF and VO2max measurements. and RPE when participants used experimental END between the three facial types. Analysis of Variance (ANOVA) with one factor (OneWay) was used. The Wilcoxon test was performed for comparisons between two periods in relation to a variable of interest. All results were considered significant at the 5 % significance level (p < 0.05). The computer package used was the Statistical Package for Social Sciences (SPSS) 26.0 for Windows (Statistical Software).
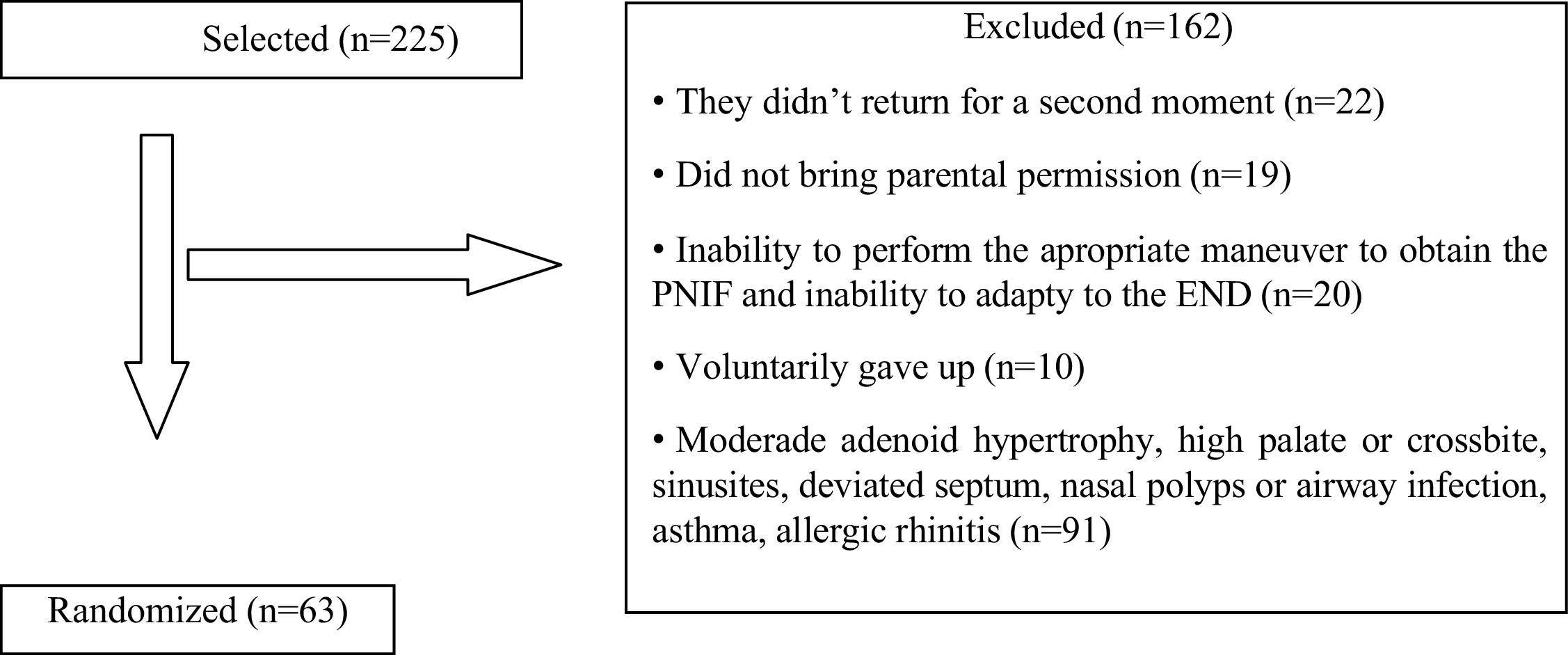
ResultsA total of 225 adolescents were initially selected. Of these, 63 met the inclusion criteria, 32 boys and 31 girls. A total of 162 participants were excluded. There was good acceptance and tolerability among participants regarding the use of the external nasal dilator and there were no complications or dependencies, even due to the study design. Fig. 3 shows the reasons for exclusion and the total number of adolescents randomized.
Regarding facial type, 20 (31.8 %) adolescents had a dolicofacial facial type, 22 (34.9 %) had a braquifacial facial type, and 21 (33.3 %) had a mesofacial facial type.
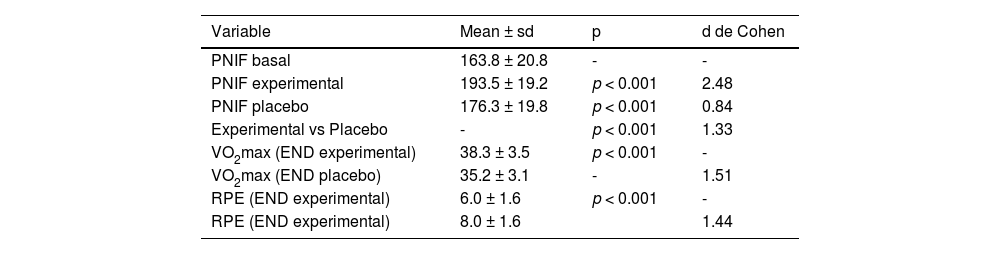
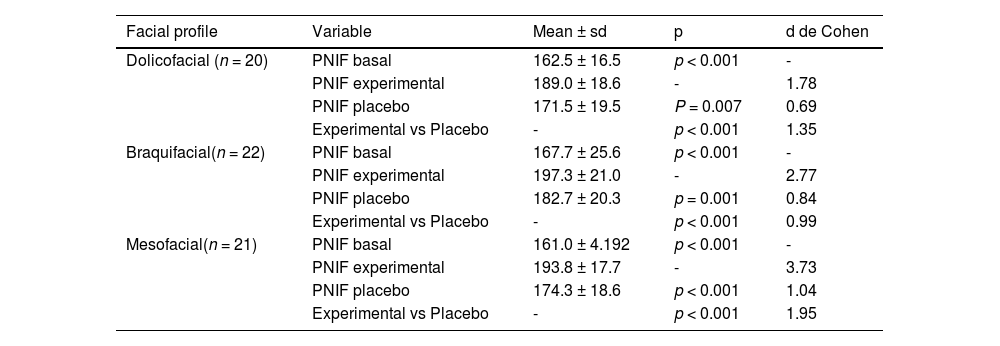
Table 1 presents the descriptive and comparative means of the baseline, experimental, and placebo PNIF measurements, as well as the VO2max and RPE values observed in the 63 adolescents during the experimental and placebo conditions of END use. The results show that there was a significant difference (p < 0.05) between the baseline, experimental, and placebo PNIF measurements. Regarding VO2max, the results reveal a significant difference (p < 0.05) when the participants used the experimental END compared to the placebo. Regarding RPE, a significant difference (p < 0.05) was also observed when the participants used the experimental END compared to the placebo. The results in Table 2 present the analysis of the participants grouped by facial profile (dolicofacial, braquifacial and mesofacial), comparing the PNIF parameters in the basal, experimental and placebo conditions.
. Descriptive and comparative analysis between baseline, experimental and placebo PNIF measurements, experimental and placebo VO2max and experimental and placebo RPE.
. Descriptive and comparative analysis between baseline, experimental and placebo PNIF measurements according to the dolicofacial, braquifacial and mesofacial profiles.
PNIF: peak nasal inspiratory flow, VO2max: maximum oxygen uptake, RPE: rating of perceived effort. sd: standard deviation. p: probability of significance of Student's t-test for paired samples. Cohen's d: effect size.
PNIF: peak nasal inspiratory flow, sd: standard deviation. p: probability of significance of the Student's t-test for paired samples. Cohen's d: effect size. Kappa and 95 % confidence interval (CI): dolicofacial = 0.90, 95 % CI (0.79; 1.00); braquifacial = 0.82, 95 % CI (0.68; 0.95); mesofacial = 0.83, 95 % CI (0.69; 0.97).
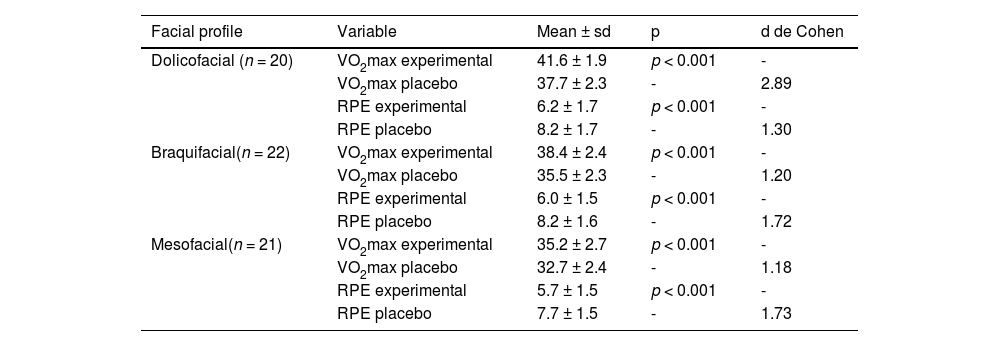
The results in Table 3 shows the analyses and comparisons between the VO2max and RPE parameters and the three facial types (dolicofacial, braquifacial and mesofacial).
. Descriptive and comparative analysis between the VO2max and RPE measurements,experimental and placebo according to the dolicofacial, braquifacial and mesofacial profiles.
VO2max: maximum oxygen uptake, RPE: rating of perceived effort. sd: standard deviation. p: probability of significance of Student's t-test for paired samples. Cohen's d: effect size.
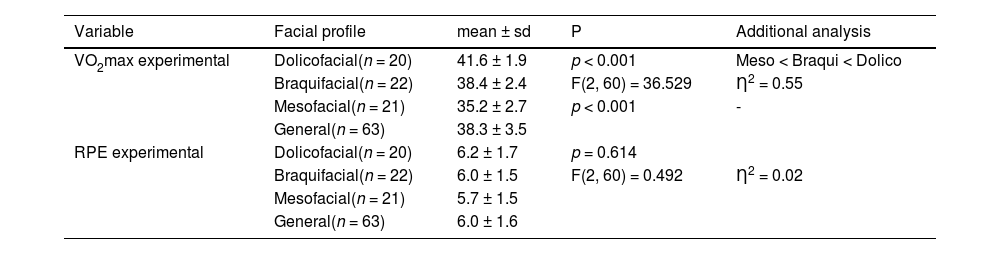
Table 4 revealed a significant difference (p < 0.05) between facial types in the measurement of VO2max using the experimental END. Participants with a dolicofacial profile had a significantly higher mean VO2max (41.6 ± 1.9 mL/kg.min⁻¹) compared to the other profiles. In addition, participants with a braquifacial profile had a significantly higher mean VO2max (38.4 ± 2.4 mL/kg.min⁻¹) than participants with a mesofacial profile (35.2 ± 2.7 mL/kg.min⁻¹). On the other hand, the analysis of the RPE measurements between the three facial types did not reveal significant differences (p ≥ 0.05).
. Descriptive and comparative analysis between the dolicofacial, braquifacial and mesofacial profiles regarding the VO2max and experimental RPE measurements.
| Variable | Facial profile | mean ± sd | P | Additional analysis |
|---|---|---|---|---|
| VO2max experimental | Dolicofacial(n = 20) | 41.6 ± 1.9 | p < 0.001 | Meso < Braqui < Dolico |
| Braquifacial(n = 22) | 38.4 ± 2.4 | F(2, 60) = 36.529 | Ƞ2 = 0.55 | |
| Mesofacial(n = 21) | 35.2 ± 2.7 | p < 0.001 | - | |
| General(n = 63) | 38.3 ± 3.5 | |||
| RPE experimental | Dolicofacial(n = 20) | 6.2 ± 1.7 | p = 0.614 | |
| Braquifacial(n = 22) | 6.0 ± 1.5 | F(2, 60) = 0.492 | Ƞ2 = 0.02 | |
| Mesofacial(n = 21) | 5.7 ± 1.5 | |||
| General(n = 63) | 6.0 ± 1.6 |
VO2max: maximum oxygen uptake, RPE: Rating of perceived effort. sd: standard deviation. p: probability of significance from the Student's T test for paired samples. F = Analysis of Variance (ANOVA) with 1 factor. Ƞ2: effect size = multiple comparisons using Bonferroni correction.
DiscussionThe present investigation explored the effects of END, considering facial types, on the cardio-respiratory capacity and nasal function of adolescent futsal players. As we hypothesized, the use of experimental END provided an important improvement in maximum oxygen uptake (VO2max), in addition to an increase in PNIF values and a reduction in RPE, verified after the cardio-respiratory test. These results are in line with previous studies.1–3 However, participants in the dolicofacial type had a higher mean VO2max when compared to the other types (braquifacial and mesofacial) when using the experimental END. The END used in the present study can be used by coaches and health professionals if the objective is to improve aerobic exercise performance or improve nasal function in adolescents.
Nasal function plays a crucial role during exercise, especially for athletes. With increasing exercise intensity, there is a rapid increase in minute ventilation, leading to a transition from nasal to oral breathing, in order to reduce resistance to air flow.14 Seto-Poon et al.15 observed a prolongation of nasal breathing during exercise and a reduction in nasal inspiratory resistance at rest in healthy adults who used the END.
Despite this, no increase in aerobic exercise performance was observed in trained adult cyclists when using the END or internal nasal dilator and control.16 We observed in adolescent athletes a significant improvement in VO2max in the experimental group compared to placebo. Similarly, Griffin et al.7 observed a significant reduction in perceived effort, heart rate, ventilation and improvement in oxygen consumption during submaximal exercise in adults, compared to placebo, results very close to those found by Dinardi et al.2 in adolescents. The different results between the studies may be due to the different methodologies used, the population analyzed, among other factors. To date, no similar study has been identified in the literature, which used a paired, randomized, double-blind, placebo-controlled sample to evaluate the effects of END according to facial type. This information strengthens the concept that data on the topic is incipient, especially in adolescentes.17,18
The bones of the skull (frontal, nasal and maxillary) contribute to the skeletal structure of the nose.19 Considering the type of nose, Ochi et al.20 observed that the effects of END are influenced by nasal type, however the present study is the first to evaluate the effects of END on VO2max, RPE and nasal function, considering facial types. It was demonstrated that the dolicofacial type (vertical growth direction greater than horizontal, longer face with a long, oval head) showed a significant difference (p < 0.001) in VO2max when using the END, compared to the other types (braquifacial and mesofacial). The dolicofacial typology is associated with more pronounced changes, often related to respiratory obstruction due to the reduced dimensions of the upper airways.12 Therefore, it is reasonable to conclude that future studies and coaches should consider the influence of facial type on the effect of END on VO2max. Despite this, RPE did not show a significant difference between facial types. Despite the benefits of RPE, the limitations and disadvantages of this tool in the context of exercise and sports science are complex and beyond the scope of this work to be discussed in depth.21
We assessed nasal function using the PNIF, a low-cost, simple, practical instrument with the power to discriminate nasal obstruction in children, adolescents and adults.22 Recently, PNIF has been used as a tool to evaluate the effectiveness of nasal dilators in adults and adolescents who practice physical exercises.1–3,23,24 Similarly, we assessed nasal flow or patency before physical exercise without END, with END or placebo in adolescents. The data from this study confirmed that END promotes an increase in the cross-sectional area of the nasal valve, resulting in a reduction in nasal resistance. These changes can positively influence essential physiological functions such as heating, filtration, humidification and dynamic airflow regulation. Such functions are especially relevant for both the general population and athletes, contributing to quality of life, sleep quality and recovery after training.14
The present study has limitations and strengths that deserve to be highlighted. The results obtained are specific to the protocols adopted, therefore, the use of different evaluation methods, populations, brands of nasal dilators and mechanisms of action (external or internal) can influence the outcomes, such as performances in aerobic exercise and improvement in nasal function. In the present study, although a reference method was used to estimate VO2max, the assessment was performed in the field, and not in the laboratory, where cycle ergometers or treadmills are typically used. Although laboratory studies are essential tools for scientific research in sport, the principle of training specificity highlights that a cycle ergometer does not reproduce, in all variables, the conditions of the race. For example, in a laboratory environment, there is no energy expenditure related to maintaining balance while running, aerodynamic resistance, terrain irregularities or the influence of ambient temperature, factors present in field tests. Furthermore, on a treadmill, the floor moves, while on a track or court the athlete moves on a fixed surface, which considerably alters muscle mechanics in terms of coordination, balance and application of forces.
Another point to consider is that direct measurement in the laboratory is relatively complex and expensive. For this reason, over time, several indirect tests have been developed to estimate VO2max. In the present study, we used Léger's indirect field test to estimate participants' VO2max,10 where there is a strong correlation (0.92) in the literature with direct analysis of VO2max.25 Therefore, the results must be interpreted with caution when extrapolating. We recommend that future studies investigate the effects of END using different aerobic capacity assessment protocols, as well as different populations and facial profiles, including healthy individuals and people with conditions such as allergic rhinitis. The facial type was assessed using the facial ectoscopy method (observation), whose main limitation lies in subjectivity, as it depends directly on the experience, skill and perception of the evaluator. This characteristic can result in inconsistent or inaccurate interpretations, especially in cases where facial features are less evident. However, we recorded inter-rater agreement rates of 0.90, 0.82 and 0.83 for the dolicofacial, braquifacial and mesofacial types, respectively. If there was a discrepancy between the evaluators, the study coordinator would be consulted to allocate the participant to a group. Furthermore, we adopt strict standardization criteria and pay attention to external factors, such as posture, lighting and excess weight, to minimize possible compromises in classification accuracy. To overcome these limitations, we suggest that future studies combine ectoscopy with complementary methods, such as radiological examinations or photogrammetry,26 to increase the objectivity and accuracy of the data. Although our study presents a different protocol configuration, it offers valuable information to enrich this discussion. Thus, coaches and health professionals interested in improving cardio-respiratory performance and nasal function may consider using the proposed resource as an effective tool to achieve these goals.
ConclusionThe present study demonstrated that the use of END resulted in a significant improvement in VO2max, in addition to an increase in PNIF values and a reduction in RPE after cardio-respiratory testing in adolescent futsal players. In particular, participants with a dolicofacial profile presented higher VO2max averages compared to the other profiles (braquifacial and mesofacial) when using the experimental END. Future studies will be essential to consolidate the effectiveness of END, considering different facial types and specific populations, including individuals with chronic diseases such as allergic rhinitis or asthma.
Indication of contributions of each authorCHSF: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. COL: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – review & editing. CCI: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing review & editing. RRD: Conceptualization, Data curation, Software, Visualization, Writing – original draft, Writing – review & editing. CRA: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing.
Data availabilityThe data that support the findings of this study are available from the corresponding author, xxx, upon reasonable request.
FundingThe author(s) reported there is no funding associated with the work featured in this article.
No potential conflict of interest was reported by the author(s).