The aim of this study was to verify whether the mean percentage of oxygen use in the aerobic-anaerobic transition phase or isocapnic buffering (IB) was lower in women with chronic fatigue syndrome (CFS) compared to healthy women, and if this variable could be used as a screening biomarker for the diagnosis of CFS
MethodsA cross-sectional study was conducted. Forty-four adult women (22 with CFS and 22 healthy) performed a cycle ergometer stress test with gas analyser (CPET). Maximum oxygen consumption (V˙O2 max), oxygen consumption at the anaerobic threshold (V˙O2 at Vt2), maximum ventilatory volume (V˙E max), time of maintenance of the isocapnic buffering phase (IB duration), and mean percentage of oxygen use in isocapnic buffering phase (% O2 use in IB) were analyzed. Data were explored from a principal component analysis. Groups were matched by propensity score to be mismatched in BMI and a comparison of means and medians was performed. A logistic regression model was built to predict the probability of CFS.
ResultsThe mean and median values of the variables obtained in the CPET was significantly higher in the healthy group compared to the CFS. In the logistic regression model, VO2 max, IB duration, and mean % of O2 use in IB were selected as predictors. The sensitivity and specificity were greater than 90%.
ConclusionThe combination of the factors V˙O2 max, IB duration, and mean % of O2 use in IB can be used as a screening biomarker for the diagnosis of CFS.
Chronic fatigue syndrome (CFS) is a serious, chronic illness characterized by persistent fatigue, worse with exertion, not improving with rest, and potentially disabling. It can affect all races and sexes of any age, with a prevalence ranging between 0.2 and 2.8% of the population, and with a female/male ratio of 3:1.1 The main characteristic of patients with CFS is poor tolerance to exercise with prolonged recovery and exacerbation of symptoms in post-exertion.2
The cause of CFS is still unknown. Genetic, endocrine, immunological or infectious factors are mixed in its etiopathogenesis and pathophysiological mechanisms.1 Although viral infections have been considered as the main trigger for the onset of the disease, there is no clear etiological mechanism in the pathogenesis.3
There is no sufficiently validated marker to diagnose the disease, nor is there a treatment that could be considered curative.1 Although diagnostic molecular biomarkers have been sought in multiple research areas, none of them have shown sufficient consistency.3
Gas analysis during a stress test (CPET) generates data that are very useful for assessing functional capacity, as has been observed in patients with cardiovascular or respiratory disease4 and also in the CFS, where the double stress test performed with an interval of 24 hours is used. The study variables try to observe the percentage deterioration of the patient with CFS, with respect to healthy people, whose variation in each of them is minimal or does not exist.5-7
The identification of biomarkers that may contribute to the etiopathogenesis of the disease could reveal a new diagnostic and therapeutic approach. For this reason, the main aim of this study was to verify whether the mean percentage of oxygen use in the aerobic-anaerobic transition phase or isocapnic buffering (IB) was lower in women with CFS compared to healthy women, and if this variable, together with other respiratory variables obtained in CPET, could be used as screening biomarkers for the diagnosis of CFS.
Material and methodsParticipantsA cross-sectional study was conducted. A total of 44 adult females were selected (48.00 ± 7.06 years): 22 with CFS and 22 healthy. Patients with CFS were defined according to the criteria proposed by,8 with more than five years of disease duration and diagnosed by the Rheumatology Service of the Santa Maria University Hospital of Lleida. The healthy patients formed the control group, had no previous pathologies and were active, since they performed physical activity between one and three times a week. All participants signed an informed consent before starting the assessments. This study was part of a research project approved by the Ethics and Research Committee of the Arnau de Vilanova University Hospital of Lleida in 2020.
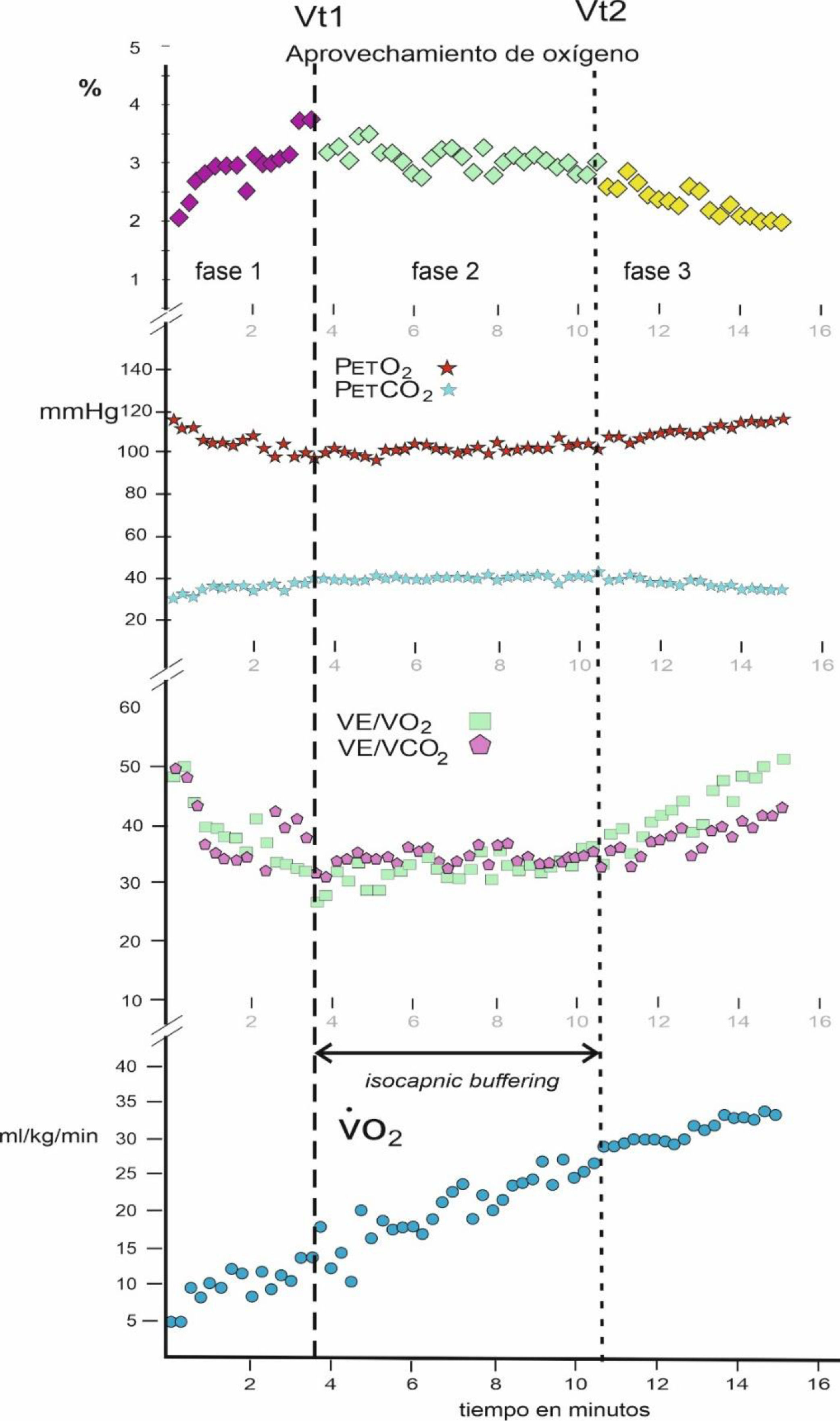
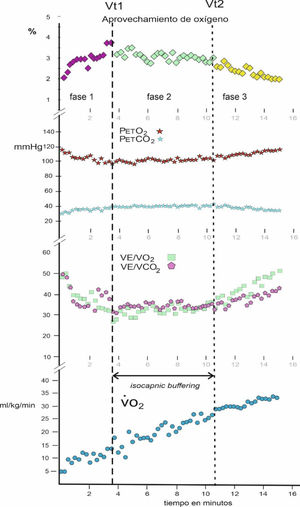
ProceduresA serological test was applied to all the participants to detect whether they were positive for the COVID-19 virus. Subsequently, a CPET stress test was applied to them on an Ergoselect 100 cycle ergometer (Ergoline, Germany). After 2 min of rest while seated on the cycle ergometer, their resting values were recorded and then they started pedaling with a resistance of 25 W and a cadence between 55 and 65 rpm, increasing 25 W every 2 min until reaching volitional exhaustion. If it was observed that due to muscular fatigue, they could not maintain the number of rpm, they were asked to stop the effort. An Ergocard CPX PRO analyzer (Medisoft, Belgium) was used for gas analysis, which was calibrated before starting each test. After CPET, the Vt1 and Vt2 thresholds were established by the ventilatory equivalents, by the partial pressures of O2 and CO2 and by the percentage of O2 use, and the IB zone was defined9 (Fig. 1).
VariablesThe variables recorded were age in decimal years (i.e., date of evaluation minus date of birth between 365.25 days), body mass index (BMI), maximum oxygen consumption (V˙O2 max), oxygen consumption at the anaerobic threshold (V˙O2 in Vt2), maximum ventilatory volume (V˙E max), the maintenance time of the isocapnic buffering phase (IB duration), and mean percentage of oxygen use in isocapnic buffering phase (% O2 use in IB). This last variable was not obtained directly from the CPET test and was subsequently calculated using the following formula:
, being the absolute value of VO2 in the laboratory equal toThe difference between both absolute values was converted into a percentage with respect to V˙E.
Statistical analysisA principal component analysis (PCA) was performed to explore the data and visualize a multidimensional reality in two dimensions through a biplot.
The CFS group was compared with the control group to detect significant differences in the respiratory variables obtained in CPET. Patients in both groups were matched by propensity score to minimize the possible confounding effect of age and BMI. The comparison of the groups was carried out with the paired and unpaired sample. The assumption of normality was verified from the Shapiro-Wilk test. When this assumption was not rejected, the mean, the standard deviation, the 95% CI of the difference between two means, and the 95% CI of Cohen's d were estimated. When the assumption of normality was rejected, the median, the interquartile interval, and the 95% CI of the difference between two medians were calculated.10 In the latter case, Cohen's d (without CI) was estimated from the z-value obtained in the Mann-Whitney U test and the formulas proposed by.11
A parsimonious logistic regression model was constructed to predict the probability of suffering CFS from the variables V˙O2 max, IB duration, and mean % of O2 use in IB.
The principal component analysis was performed in RStudio version 1.4.1717 software (RStudio, PBC, Boston, MA). All other statistical analyses were performed in Stata/IC version 17.0 software (StataCorp, College Station, TX).
ResultsThe mean oxygen use in IB between the two thresholds Vt1 and Vt2 was related to the mean of the ventilatory oxygen equivalent in IB (V˙E/V˙O2) for each subject, obtaining a correlation coefficient of r = .987 for N = 44.
Table 1 shows the comparison of the CFS group with respect to the control in the different variables of the study. A significant difference was detected in BMI when the sample was unpaired. In contrast, with the matched sample this difference became non-significant, which justified the propensity analysis. In all the respiratory variables measured in the CPET, a significant difference was found, with a large effect size greater than 0.80 in absolute values.
Comparison between groups with matched and unmatched sample.
CI = confidence interval; M = mean; SD = standard deviation; Mdn = median; IQI = interquartile interval.
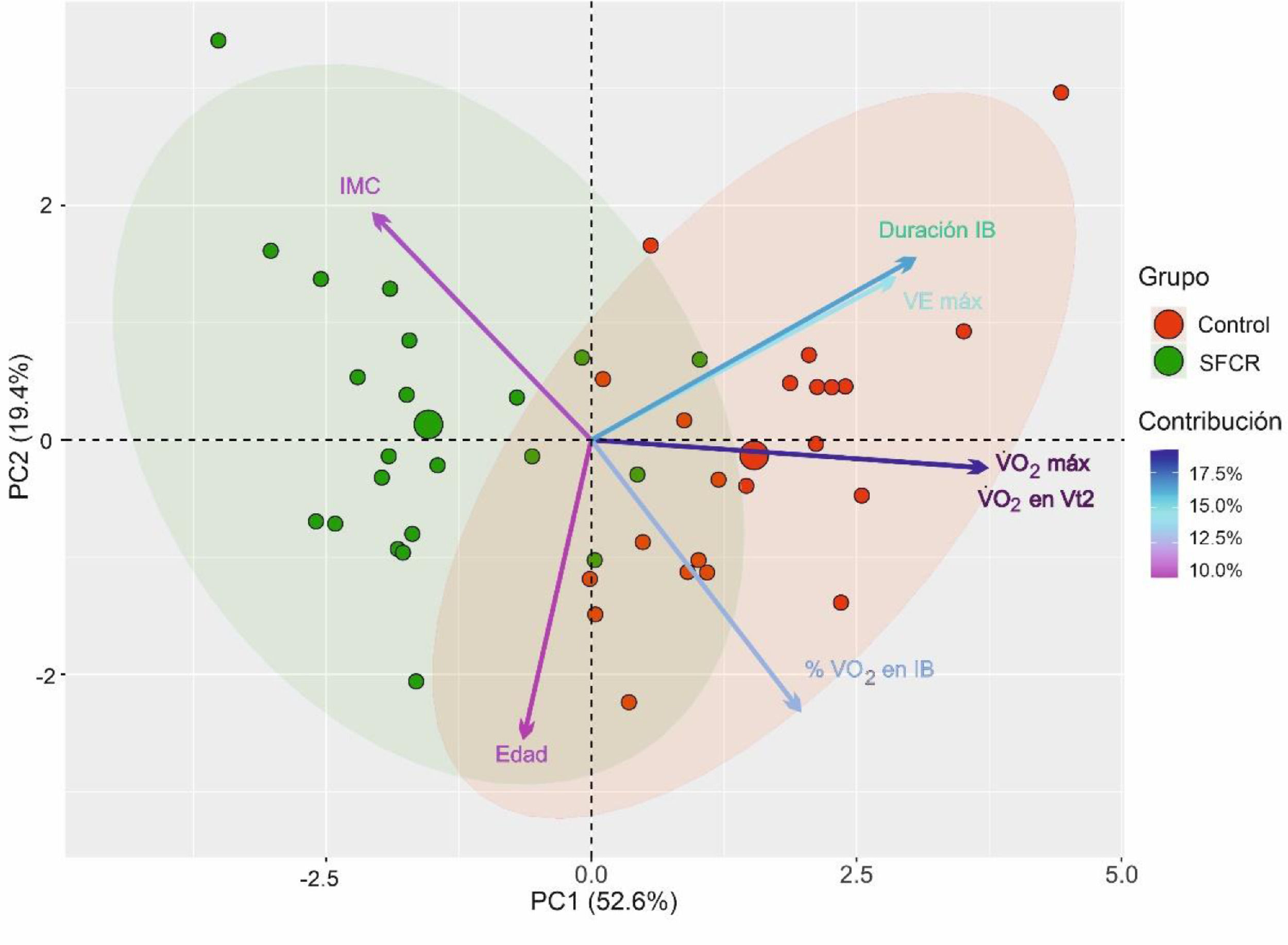
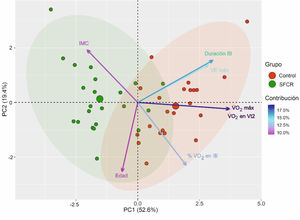
Fig. 2 shows the PCA. The first two components out of a total of seven had an eigenvalue greater than 1 and explained 72.05% of the total variance of the data. In the first principal component (PC1), the variables V˙O2 max, V˙O2 at Vt2, IB duration, and V˙E max had a greater weight. In contrast, in the second principal component (PC2), the variables age, mean % of O2 use in IB, and BMI had a greater weight. The direction of the arrows revealed that the variables V˙O2 max and V˙O2 at Vt2, on the one hand, and V˙E max and IB duration, on the other hand, were correlated and provided redundant information, so they were excluded from the subsequent logistic regression analysis. Finally, the 95% confidence ellipses for each group and their center revealed that (a) the CFS group had higher BMI; that (b) the control group had higher V˙O2 max, V˙O2 at Vt2, V˙E max, IB duration, and mean % of O2 use in IB; and that (c) both groups had similar age. These results confirm those presented in Table 1.
The predictor variables included in the logistic regression model (V˙O2 max, IB duration, and % O2 use in IB) statistically significantly predicted (χLR2 = 42.98, df = 3, p < .001) the binary outcome (group: 0 = control, 1 = CFS). This model explained between 57.3% and 83.1% of the uncertainty in the data (pseudo-RMcFaddenAdj2 = .573, pseudo-RCox−Snell2 = .623, pseudo-RNagelkerke2 = .831); had an area under the ROC curve (AUC = .973, 95% CI [.880, .999]) and a p-value in the Hosmer-Lemeshow test (p = .993) close to 1; and had a high and balanced sensitivity and specificity for detecting CFS cases and controls, respectively (Se = 90.9% and Sp = 90.9% for a cut-off point of .46). All these results indicated a good fit and a good classificatory capacity of the estimated model. Furthermore, this model had a mean variance inflation factor of less than 10 (mean VIF = 2.14), indicating an absence of collinearity between predictors.
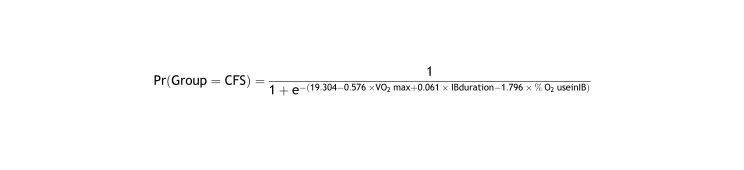
The following logistic function was defined from the b coefficients of the estimated model:
From this equation, the probability of suffering CFS was predicted for different values of the predictor variables (Table 2).
Prediction of the probability of suffering CRF (expressed as a percentage) for different values of V˙O2 max, IB duration, and mean % of O2 use in IB.
In the results, it was observed that there is a very high correlation (r = .987) between the mean of the ventilatory oxygen equivalent in IB (V˙E/V˙O2) with the mean of the % use of oxygen in that phase, which shows a statistical validity for its interpretation, avoiding the large data dispersion of the ventilatory equivalent O2.
One of the objectives of this study was to verify whether the mean percentage of oxygen use in IB was lower in women with CFS compared to healthy women. This hypothesis was confirmed in both matched and unmatched samples, with a large effect size (Cohen's d > 0.80), but lower than the other respiratory variables compared.
In the same laboratory that carried out the present study and applying the same protocol described to 26 female athletes of good endurance level, with a mean age of 38.23 ± 9.4 years, a V˙O2 max of 41.80 ± 6.62 ml/kg/min and a mean % of O2 use in IB with respect to V˙E of 4.02 ± 0.5% were obtained. However, having a better percentage is not always synonymous with reaching higher oxygen consumption (r = .50). There may be people with a high percentage of oxygen use, even higher than average and with a relatively low oxygen consumption. This suggests that both V˙O2 max, IB duration, and mean % of O2 use in IB should always be studied together.
In a CPET, the decrease in the mean % of O2 use in IB can be interpreted as an alteration of mitochondrial function in energy provision.9 Currently, a possible mitochondrial dysfunction at the molecular level is being investigated, either due to an inadequate number of mitochondria in the cell or due to an alteration in mitochondrial function, which would lead to a reduction in the efficiency of oxidative phosphorylation and a reduction in the production of the ATP.12-14
Regarding the mean duration of IB in women with CFS was 3.09 min (i.e., 3 min and 5 s), while in healthy women it was 6.42 min (i.e., 6 min 25 s). Consequently, healthy women double the average duration time of the aerobic-anaerobic transition phase.
From building a logistic regression model with V˙O2 max, IB duration, and mean % of O2 use in IB as predictor variables and having or not having chronic fatigue as response variable, an equation with acceptable classification power was obtained, since both the sensitivity and the specificity of the model had a high level greater than 90%.
Regarding the study of the mean percentage of oxygen use in IB phase, the need to analyze in greater depth the possible metabolic basis of mitochondrial dysfunction is confirmed, for which the application of unique techniques such as non-directed metabolomics techniques is proposed, which allow us to discern a footprint or metabolic pattern of chronic fatigue, as well as the potential detection of specific biomarkers of said condition.
As limitations of the study, our results should be applied to a larger population group and adding the male sex. The regression for the analyzed factors does not allow to totally exclude that there are other residual factors that are not valued.
ConclusionThe study of the combination of the factors V˙O2 max, IB duration, and mean % of O2 use in IB support the validity of these as a screening biomarker for the diagnosis of CFS with a predictive power in our sample of over 90%.
The results obtained make it possible to propose the confirmatory screening of the CFS with a single laboratory test, and not with the two sessions of the most commonly used procedure currently4,6 with the importance that this stress reduction factor has for affected population.
The authors would like to thank the Santa Maria University Hospital of Lleida, the IRB of Lleida, the Sports Medicine Center of Lleida, the INEFC Lleida, the University of Lleida, and the Ekke Sports Center for their collaboration.
Funding: This study has been possible thanks to funding from the Andromeda Foundation and the Diputació de Lleida (Lleida County Counci).