The aim of these studies was to evaluate the effects of regular volleyball training on salivary SIgA and alpha-amylase in female children and adolescents.
Material and methodIn the cross-sectional study, 115 female children (12.6±2.2 years) participating in a Volleyball training program were classified as beginners, intermediate, and competitive level. The children were evaluated regarding caries index, body mass index (BMI), cardiorespiratory fitness, and countermovement jump. In the longitudinal study: 54 girls (intermediate and competitive groups) trained for 8 weeks and were re-evaluated at weeks 4 and 8.
ResultsThe SIgA secretion rate and alpha-amylase activity did not present correlations with training category, age, caries index, or training status. A weak positive correlation was detected between IgA secretion rate and BMI (r=0.20, p<0.05). After 8 weeks of training, VO2max (p<0.005) and jump height (p<0.005) improved in the competitive girls. No differences were observed in salivary secretion rate, SIgA concentration and secretion rate, or alpha-amylase activity at weeks 4 and 8.
ConclusionWe concluded that despite improving physical fitness, 8-weeks of recreational volleyball training are not able to improve salivary SIgA secretion or alter alpha-amylase activity in female children and adolescents.
The practice of regular physical exercises at moderate intensity or recreational sports training is associated with positive modulation of secretory immune responses and decreased stress in adult populations.1–5 Salivary secretory Immunoglobulin A (SIgA) is the most abundant antibody in saliva and plays important roles in the defense of the oral cavity against dental caries, periodontal diseases, and oral and upper respiratory tract infections.5–7 Clinical data have suggested that regular exercise practice and increased physical fitness were correlated to increased SIgA levels and improved oral health.1,3,8,9 In this way, improving physical fitness may have positive effects on oral immunity.
Salivary SIgA secretion is driven by the autonomic nervous system and its secretion can be modulated by physical exercise.5,10,11 A single bout of moderate exercise (75% VO2max) is able to improve oral mucosal immunity over 24h, increasing salivary secretion of SIgA.12 Other authors demonstrated that aerobic exercise training programs could increase resting SIgA secretion in adults.3,4 A study in female undergraduate students demonstrated that students engaged in regular team sports training (in season) presented lower levels of stress markers and increased levels of SIgA compared to the “off season” students.1 While acute and regular moderate exercise can improve salivary secretion, low levels of physical activity are associated with impairments in the immune response in adults.2,5 However, little is known about the effects of regular exercise practice on salivary secretion in children and adolescents.
Salivary alpha amylase is a digestive and antimicrobial enzyme that has been used to monitor the stress balance and activation of the sympathetic nervous system.13,14 Some studies have suggested that amylase levels are modulated by stress, body mass index (BMI), and physical fitness in children and adults.1,15,16 In adult men, muscle power and cardiovascular fitness are associated with better regulation of the autonomic nervous system and lower secretion of salivary amylase.15 Female undergraduate students engaged in regular team sport training demonstrated reduced levels of stress and salivary alpha-amylase secretion.1 A low cardiorespiratory fitness level was also associated with increased sympathetic activity and salivary alpha-amylase concentration in school children.16 Higher levels of alpha-amylase are associated with increased BMI and emotional stress in pre-school children.11,16 However, other authors did not find differences in salivary alpha-amylase concentration between normal and overweight children aged 5–12 years.18 Currently, it is not known whether physical training could modulate resting alpha-amylase secretion, since it may improve autonomic nervous system modulation and body mass index control.14,19
Although the benefits of regular physical activity have been reported in adult and elderly populations,3,4,20,21 the effects of regular practice of exercise on salivary secretory immunity in children and adolescents are not yet clear. A study in African children reported that overweight and obese children presented decreased cardiorespiratory fitness, lower salivary SIgA concentration and secretion rate, and increased levels of salivary alpha-amylase.16 Since engagement in physical activities improves immunity defenses in adult populations,3,5–21 it may also have some beneficial effects on children and adolescents. Therefore, the objectives of the present studies were to determine whether engagement of female children and adolescents in volleyball training could improve secretory immunity in saliva and the effects of changing physical performance on SIgA and alpha-amylase secretion.
Material and methodsStudy designThis study was divided into two steps:
- •
Cross-sectional study: a transversal study on the association between saliva flow rate, SIgA concentration, SIgA secretion rate, and alpha-amylase concentration with BMI and physical fitness;
- •
Cohort study: a longitudinal study of the effects of 8 weeks of volleyball training on salivary secretory immunity.
The study was approved by the Research Ethics Committee Involving Human Beings of the State University of Londrina (protocol no. 821.804) and followed the principles of the Helsinki Declaration (2013).22All parents or legal guardians, as well as the children and adolescents, gave their written informed consent.
In the cross-sectional study, 148 girls were included, ranging in age from 7 to 17 years. The girls trained in the Project of School Sports of the Department of Sports of the Municipality of Cambé – Paraná, Brazil. The samples were collected during a previous study (da Silva et al., 2019)23 of our research group that evaluated their salivary inflammatory profile. The girls were evaluated in the first month of training (August, 2015) after the July (2015) vacation period. During the vacation period (30 days), girls and parents reported no participation in systematic training and sports activities. In the initial evaluation, we included all the girls who presented a free and informed consent signed by the parents and themselves. We excluded girls who presented chronic inflammatory diseases, oral mucosal lesions, continued use of medication, diabetics and other chronic inflammatory diseases, bronchitis and asthma, recent use of corticosteroids (less than 6 months), and those who donated an insufficient sample volume for amylase analysis and could not perform the physical tests.
In the cohort study, the girls were re-evaluated after four and eight weeks of training. Only girls who donated a sufficient volume of saliva and were regularly attending training sessions were included in the final evaluation, with up to two absences in training sessions allowed during the investigation period. In addition, a control group of 40 girls (12.4±2.17 years, normal weight) from local schools, not participating in sports training, were evaluated for salivary SIgA and amylase at baseline and after 8 weeks.
ParticipantsThe initial evaluation (Pre) was used in the cross-sectional study analysis. The girls were submitted to physical evaluation, saliva sampling, the countermovement jump test (CMJ), and 20m shuttle run test (Léger test). Regarding their previous participation in the School Sports Project, the girls were classified into three categories:
- •
Beginners (without previous experience): the girls had no previous experience in volleyball practice and were not engaged in other sports activities. These girls had less than 3 weeks of training.
- •
Intermediate group: minimum time of three months and maximum of 12 months of engagement in volleyball training. The girls had participated in the sports program since the previous semester, but did not participate regularly in competitive events.
- •
Competitive group: minimum of 13 months of training. The girls in the competitive group had participated in volleyball training for more than two school semesters and were engaged in local and state school game competitions.
The saliva samples were collected as previously described by da Silva et al.23 The saliva samples were collected at rest before physical tests and training commenced, 1h after their last meal, between 09:00 and 10:00 a.m. and 2:00 p.m. and 3:00 p.m., according to the training schedule. The girls were asked to brush their teeth 1h before saliva sampling to eliminate excess toothpaste residues from saliva. The girls were instructed to rinse their mouths with drinking water for 1min prior to saliva collection, and then salivate spontaneously into sterile graduated collection tubes for 2min. The salivary flow rate was assessed by measuring the volume of secreted saliva per minute (ml/min). Samples were frozen at −20°C prior to use. Menstrual phase was not controlled because it has been demonstrated not to influence secretion of SIgA and other antimicrobial proteins at rest.24 Unstimulated whole saliva was used since stimulated salivation could increase saliva flow rate and alpha amylase secretion or cause bias in the SIgA secretion rate calculus.25
Enzyme-linked immunosorbent assay for determination of salivary IgAThe saliva samples were centrifuged at 4000×g for 5min for sedimentation of cell debris. Supernatants from the saliva samples were diluted at 1:1000 in phosphate buffered saline (PBS, pH 7.2). Next, the samples were submitted to an ELISA assay for determination of the salivary SIgA concentration, using commercial kits as recommended by the manufacturer (A88-102P, Bethyl Laboratories, Montgomery, USA). The SIgA concentration was expressed in μg/ml saliva and the secretion rate was determined by IgA secretion per minute (μg/min).26
Detection of saliva alpha-amylaseThe concentration of salivary alpha-amylase was determined with a commercial enzyme kit (Phadebas Pharmacia Diagnostica, Uppsala, Sweden), as recommended by the manufacturer.
Physical evaluationHeight in centimeters was measured using a stadiometer and weight was evaluated using a digital scale (Omhron HBF 514C, Omhron Health Care do Brasil, São Paulo, Brazil). The body mass index was calculated using the formula BMI=mass (kg)/height2 (m). The girls were classified according to BMI into: very underweight, underweight, normal, overweight, and obese according to the criteria adopted by the World Health Organization for girls between 0 and 19 years of age.27
The oral examination was performed by one trained Periodontist and two Pediatric dentists, (Kappa=0.99). An oral examination was performed using artificial light, spatulas, dental mirrors, and probes. The index of decayed, missing, and filled teeth for permanent teeth (DMFT) was determined according to the criteria described by the World Health Organization.28 During the oral examination, the presence of soft tissue lesions and spontaneous gingival bleeding were also recorded.
20m shuttle run test (Léger test)The maximum oxygen consumption (VO2max) was estimated using the maximum progressive test developed by Léger and Lambert in 1982 and modified by Léger et al. in 1984.29 The formula described by Léger et al. in 1988 for children aged 8–19 years30 was used to calculate estimated cardiorespiratory fitness.
Countermovement jump testThe girls were familiarized with the countermovement jump test prior to data collection. The vertical jumping test with countermovement was performed on a contact mat (Smart jump, Fusion Sports, Summer Park, Australia),31 with three consecutive jumps with a 1min interval. The highest jump height in three attempts was recorded. For the test, the children were instructed to stand erect and, when a light signal came on, crouch quickly with their knees bent at approximately 90° and immediately jump with their legs extended. The arms were extended during the flight phase of the jump, simulating the blocking movement at the net. The phase of menstrual cycle was not controlled since it does not influence muscular strength in young females.32
Volleyball training programThe volleyball training practices were performed in two to three sessions per week, lasting 120–180min daily, depending on their category and previous experience in sports. The training was performed for eight consecutive weeks, and consisted of 10min of warm-up, specific technical-tactical exercises for the category, aerobic and anaerobic exercises with and without balls, simulated games, and 5min of stretching at the end of each training session. Children who were absent three or more times during the eight weeks of training were excluded from the study.
The girls evaluated in the longitudinal test completed the eight weeks of training and were submitted to the Léger test and vertical jump test in the first (Pre) and eighth weeks (Post). Saliva collections were performed at the beginning (Pre) and after four (4 weeks) and eight weeks (Post) of training.
Statistical analysisThe distribution of normality was evaluated by the Shapiro–Wilks test. Continuous variables with normal distribution are expressed as mean and standard deviation. Variables without normal distribution are expressed as medians and quartiles of 25–75%. Differences between groups were assessed using the one-way ANOVA test, with the Tukey post-test (parametric data) or Kruskal–Wallis test (non-parametric data). Categorical data are expressed in absolute frequencies and percentages and were evaluated using the Chi-square test with the Yates correction, or Fisher's exact test. The correlations between the salivary measurements and other variables were determined by the Spearman's rank-order correlation test. Two-way ANOVA was performed in the cohort study to determine the influence of time and previous experience in volleyball practice on physical performance and salivary secretion. The sphericity of data was analyzed by Mauchly's test with the Greenhouse–Geisser correction. Differences between study variables were considered significant at p<0.05. Statistical analysis was performed with Graph Pad Prism 8.0 (GraphPad software, San Diego, USA).
We considered a 50% difference in the mean SIgA concentration reported by Starzak et al.,16 for normal weight (243.0±199.2μg/ml) children, for sample size calculation. Twenty-two children per group was necessary to achieve a maximum α error of 5%, and 80% statistical power (BioEstat 5.0, Instituto de Desenvolvimento Sustentável Mamirauá, Tefe, Brazil).
ResultsCross-sectional studyIn total, 148 girls were enrolled in the volleyball program in August 2015, of which 115 (77.7%) were evaluated; 33 (22.3%) were excluded due to lack of presentation of the consent form, absence on the day of physical testing, or insufficient volume of saliva sample to perform alpha-amylase analysis.
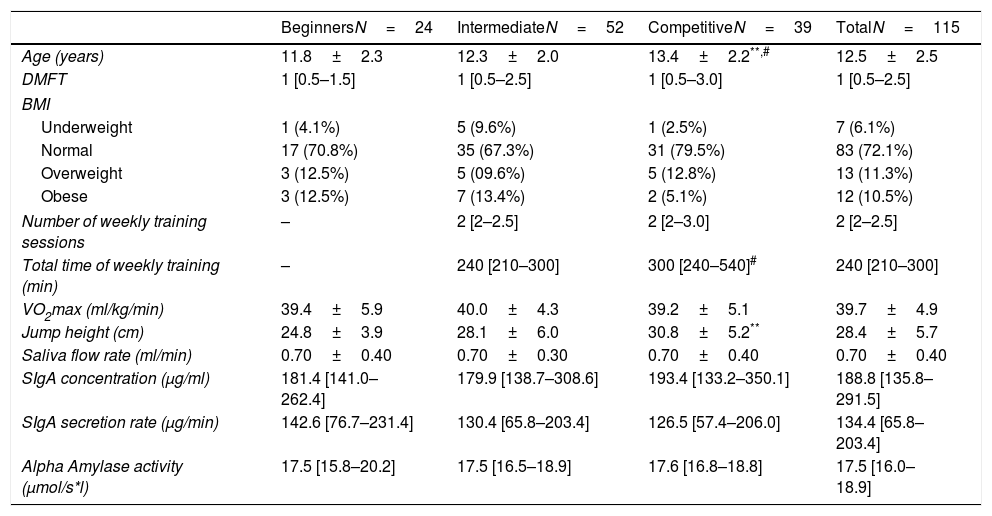
The age ranged from 7 years to 17 years. Girls in the competitive group were older than the other groups (Table 1). The frequency of girls in different BMI ranges was similar between groups (p>0.05; Chi squared test).
Characteristics of participants and salivary IgA and alpha amylase in girls with different training groups.
| BeginnersN=24 | IntermediateN=52 | CompetitiveN=39 | TotalN=115 | |
|---|---|---|---|---|
| Age (years) | 11.8±2.3 | 12.3±2.0 | 13.4±2.2**,# | 12.5±2.5 |
| DMFT | 1 [0.5–1.5] | 1 [0.5–2.5] | 1 [0.5–3.0] | 1 [0.5–2.5] |
| BMI | ||||
| Underweight | 1 (4.1%) | 5 (9.6%) | 1 (2.5%) | 7 (6.1%) |
| Normal | 17 (70.8%) | 35 (67.3%) | 31 (79.5%) | 83 (72.1%) |
| Overweight | 3 (12.5%) | 5 (09.6%) | 5 (12.8%) | 13 (11.3%) |
| Obese | 3 (12.5%) | 7 (13.4%) | 2 (5.1%) | 12 (10.5%) |
| Number of weekly training sessions | – | 2 [2–2.5] | 2 [2–3.0] | 2 [2–2.5] |
| Total time of weekly training (min) | – | 240 [210–300] | 300 [240–540]# | 240 [210–300] |
| VO2max (ml/kg/min) | 39.4±5.9 | 40.0±4.3 | 39.2±5.1 | 39.7±4.9 |
| Jump height (cm) | 24.8±3.9 | 28.1±6.0 | 30.8±5.2** | 28.4±5.7 |
| Saliva flow rate (ml/min) | 0.70±0.40 | 0.70±0.30 | 0.70±0.40 | 0.70±0.40 |
| SIgA concentration (μg/ml) | 181.4 [141.0–262.4] | 179.9 [138.7–308.6] | 193.4 [133.2–350.1] | 188.8 [135.8–291.5] |
| SIgA secretion rate (μg/min) | 142.6 [76.7–231.4] | 130.4 [65.8–203.4] | 126.5 [57.4–206.0] | 134.4 [65.8–203.4] |
| Alpha Amylase activity (μmol/s*l) | 17.5 [15.8–20.2] | 17.5 [16.5–18.9] | 17.6 [16.8–18.8] | 17.5 [16.0–18.9] |
DMFT: decayed, missing and filled teeth (caries index); BMI: body mass index; VO2max: maximum oxygen consumption, SIgA: salivary immunoglobulin A.
The mean VO2max was not significantly different between groups. However, the competitive girls presented a higher jump height compared to the beginners (Table 1).
No significant differences in saliva flow rate, SIgA concentration, SIgA secretion rate, or alpha amylase activity were detected between groups (Table 1).
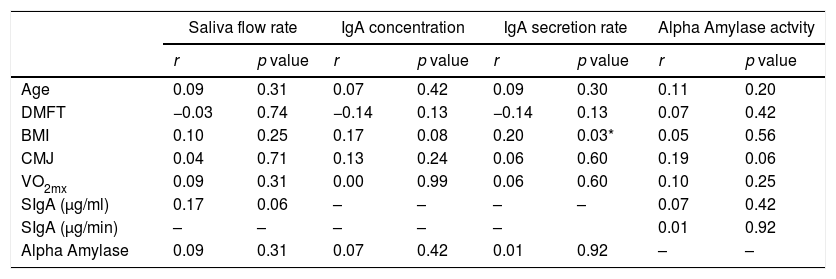
The saliva flow rate, SIgA concentration and secretion rate, and alpha-amylase activity did not present correlation with age, caries index (DMFT), or physical performance (Table 2). A week correlation was observed between IgA secretion rate and BMI score (Table 2).
Correlation between saliva flow rate, SIgA concentration and secretion rate, and Alpha amylase activity with physical characteristics.
| Saliva flow rate | IgA concentration | IgA secretion rate | Alpha Amylase actvity | |||||
|---|---|---|---|---|---|---|---|---|
| r | p value | r | p value | r | p value | r | p value | |
| Age | 0.09 | 0.31 | 0.07 | 0.42 | 0.09 | 0.30 | 0.11 | 0.20 |
| DMFT | −0.03 | 0.74 | −0.14 | 0.13 | −0.14 | 0.13 | 0.07 | 0.42 |
| BMI | 0.10 | 0.25 | 0.17 | 0.08 | 0.20 | 0.03* | 0.05 | 0.56 |
| CMJ | 0.04 | 0.71 | 0.13 | 0.24 | 0.06 | 0.60 | 0.19 | 0.06 |
| VO2mx | 0.09 | 0.31 | 0.00 | 0.99 | 0.06 | 0.60 | 0.10 | 0.25 |
| SIgA (μg/ml) | 0.17 | 0.06 | – | – | – | – | 0.07 | 0.42 |
| SIgA (μg/min) | – | – | – | – | – | 0.01 | 0.92 | |
| Alpha Amylase | 0.09 | 0.31 | 0.07 | 0.42 | 0.01 | 0.92 | – | – |
DMFT: decayed, missing and filled teeth (caries index); BMI: body mass index; VO2max: maximum oxygen consumption, SIgA: salivary immunoglobulin A.
Only 54 (46.9%) girls completed the assessments and had enough volume of saliva samples for analysis after 8 weeks of training. Three girls (05.5%) were from the beginner group, 22 (40.7%) from the intermediate group, and 29 (53.7%) from the competitive group. Considering that 24 (87.5%) girls in the beginner group did not complete the training sessions or perform the physical tests, this group was excluded from the longitudinal study.
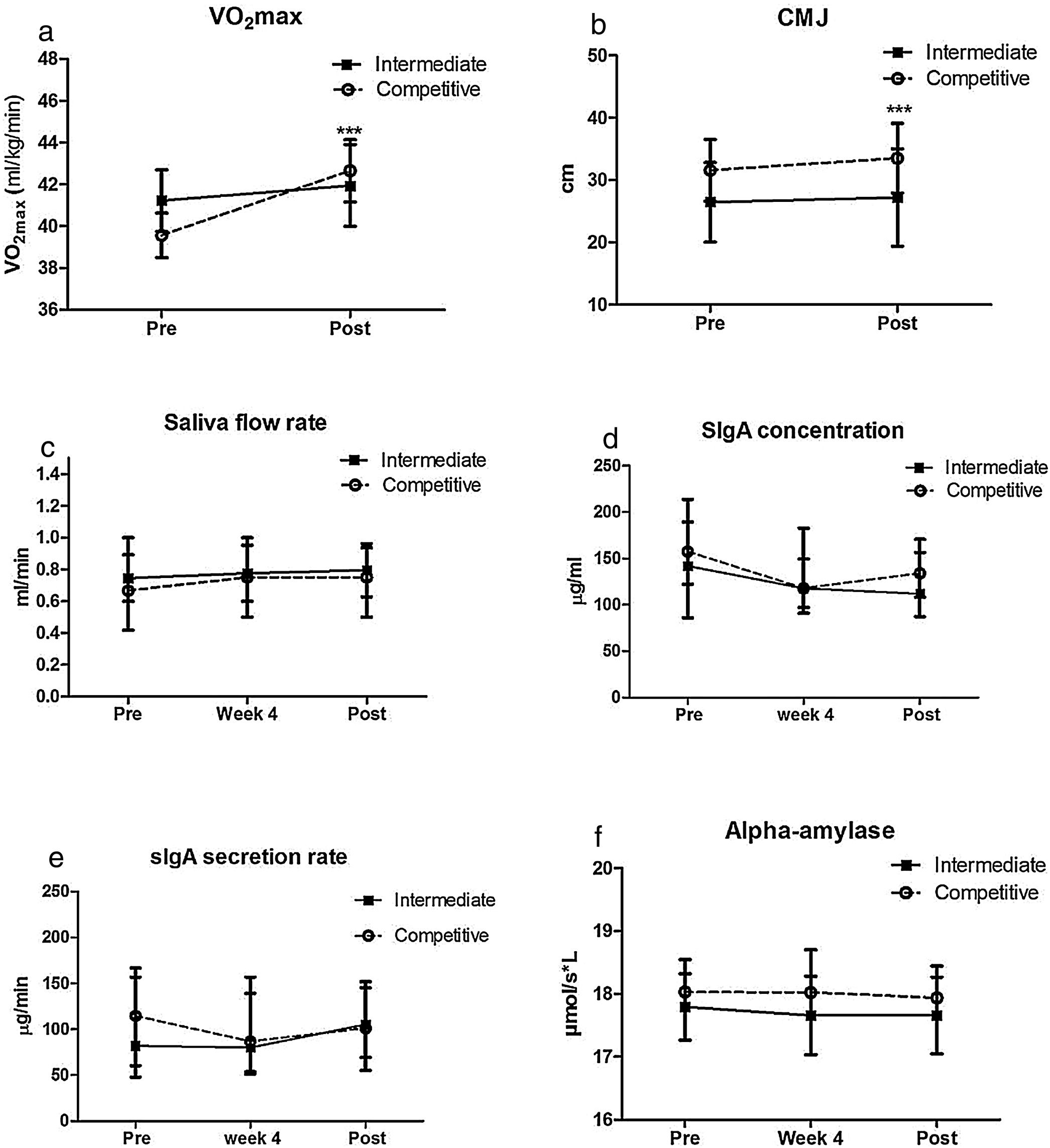
The girls in the intermediate group did not present improvement in VO2max (p=0.58, paired t test; Fig. 1a) or jump height (p=0.35, paired t test; Fig. 1b). On the other hand, girls from the competitive group presented improvement in both VO2max (p=0.003, paired t test, Fig. 1a) and jump height (p=0.002, paired t test, Fig. 1b). An effect was observed in aerobic performance only for time (p=0.009; F=7.58, two-way ANOVA). Jump performance presented group (p=0.002. F=10.13) and time (p=0.008, F=7.74, two-way ANOVA) interactions.
The levels of saliva flow rate, IgA concentration and secretion rate, and alpha amylase activity did not change significantly from Pre values to weeks 4 and Post (Fig. 1). No group or time effects and interactions were observed in either group in any salivary measurement.
The salivary variables of the beginners and competitive group were not different from a control group at pre and post (p>0.05, Kruskal–Wallis test). For the control group, the variations from pre to post for saliva flow rate were (1.19±1.23 to 0.73±0.41ml/min, p<0.05, Wilcoxon test), IgA concentration (133.6±18.3 to 169.7±46.3μg/ml, Wilcoxon test), IgA secretion rate (159.1±21.7 to 124.3±18.9μg/min, p<0.05, Paired t test), and amylase (18.1±3.0 to 18.5±2.8mmol/ml, p<0.05, paired t test).
DiscussionContrary to our hypothesis, physical fitness was not associated with regulation of salivary secretion of SIgA and alpha-amylase in female children and adolescents performing volleyball training. Improvement in physical fitness was more evident in the competitive group but was not associated with improved secretion of SIgA or downmodulation of alpha-amylase activity. This was surprising; since other studies reported salivary secretion and immunity were modulated by physical training in adults.1,4,5,20 Moreover, a previous study in young female subjects suggested that regular sports training could improve secretory immunity and reduce alpha-amylase secretion.1 We excluded two girls with active caries from the study since these could increase the salivary levels of SIgA and alpha-amylase.33 The results demonstrated that girls presented similar caries activity between study groups and the caries index was not associated with variability in SIgA and alpha-amylase secretion.
Previous studies investigating salivary levels of SIgA in school children and adolescents presented divergent results.16,34,35 One study investigated cellular and SIgA immune responses in children (aged 7–13 years) correlated with skinfold thickness, BMI, and VO2peak.35 The authors concluded that increased adiposity was correlated with impaired cellular responses but increased SIgA secretion, whereas aerobic fitness presented little effect on immune parameters.35 One study reported that obesity and low cardiorespiratory fitness were associated with reduced levels of salivary SIgA and increased alpha-amylase activity in children aged 10±1.6 years.16 However, another study demonstrated that SIgA concentration was increased in obese/overweight children (aged 6–12 years) and presented a weak correlation with BMI.34 A limitation of studies, including the current study, is missing data on maturation status and a wide variation in age (children to adolescents). A previous study investigated the relationship between age (8–17 years old), hormonal status, and grip strength with salivary IgA.36 As observed in the present study, no correlations were detected with age and strength, but a moderate correlation with dehydroepiandrosterone was observed.36 This suggests that other maturation parameters such as hormonal status, menarche, and puberty should be investigated in future studies addressing secretory immunity in female children and adolescents. Another concern regards the menstrual cycle phase, since some reports in adult women suggested that the association between menstrual phase and sexual activity may have some impact on salivary SIgA secretion,37,38 while others found no correlation of menstrual cycle with salivary secretory immunity.24,39 We expected a lower secretion rate of SIgA in overweight and obese children, since increased BMI is associated with reduced parasympathetic activity in children and adolescents.40 Contrary to our hypothesis, only a weak positive correlation was observed between SIgA secretion rate and BMI. The lower limb strength and VO2max were also not correlated with resting SIgA and alpha amylase activity. The low number of obese and overweight children observed in our study may be a limitation of the study. Another concern is that children were detrained at the initial evaluation (cross-sectional study), so training effects on mucosal immunity could be blunted. However, 8-weeks of training improved physical performance, without a significant effect on saliva secretion. Moreover, children who were not training presented similar levels of salivary SIgA and alpha-amylase and did not present significant changes in salivary secretion after 8 weeks.
In the cross-sectional study, the competitive girls presented increased jump height. This difference may be associated with differences in the age of the groups since increased maturational status was positively associated with jumping performance in youth athletes.41 Another hypothesis was that the girls from the intermediate and competitive groups were evaluated after a period of detraining (school vacations), although lower limb strength adaptations are maintained for longer periods than aerobic adaptations.42 During the training period, jump and aerobic performance significantly increased in the competitive girls, suggesting a previous state of adaptation to exercise. In young soccer athletes, a brief period of detraining impaired both strength and endurance performances, although a fast recovery in performance was observed after training began.43 Furthermore, the competitive group presented higher weekly training volume than the intermediate group, which may account for the increased physical performance in comparison to the intermediate group after 8 weeks. Indeed, increased training volume may be necessary in adolescents (>13 years) to achieve the same level of strength performance improvements as younger children.44 However, we could not monitor individual training load during the training period to draw any correlations among training volume, physical adaptation, and immune parameters. Unfortunately, controlling training load by heart rate or the lactate threshold was not feasible in this study due to the large number of participants. Using rate of perceived exertion to calculate internal training load was not appropriate as it is influenced by trainability, body mass index, and age.45,46 Thus, we made the assumption that competitive children had a higher training load due to a higher training volume, considering that the technical–tactical activities developed by both groups were similar. As expected, the girls engaged in competition events and who had trained for a longer period presented better performance improvement. However, despite the improvements in performance, no effect of training stimulus was observed on salivary secretory immunity.
A study in 10 year-old Norwegian children revealed that practice of team sports, including volleyball, was associated with increased cardiorespiratory fitness.47 Other authors demonstrated that 7 weeks of Volleyball training in female adolescent athletes improved aerobic fitness and vertical jump height.48 In male child and adolescent volleyball athletes, increased anaerobic power was associated with increased vertical jump height, compared to nonathletic subjects.49 Although improvements were observed in physical performance, the physiological stimulus seemed not to be sufficient to increase resting levels of SIgA and alpha-amylase in female children and adolescents. Indeed, aerobic performance was the main component altered by the volleyball training and a previous study demonstrated that aerobic fitness was not correlated with SIgA.35
Human studies have demonstrated that physical training and increased cardiorespiratory fitness could improve humoral immunity and are associated with improved parasympathetic balance.1,3,16,20 Moreover, increased parasympathetic activity can induce SIgA secretion, stimulating poli-Ig receptor translocation of secretory immunoglobulins into saliva.11,50 We hypothesize that during the initial evaluation, cardiorespiratory fitness was not significantly different between groups and this may account for the lack of significant differences in autonomic regulation of SIgA secretion. Indeed, increased VO2peak and VO2max were associated with better overall regulation of the autonomic nervous system in children and adolescents.51,52 Moreover, engagement in sports activity above 180min/week was associated with improved vagal autonomic regulation in children aged 10–13 years,53 so improved cardiovascular fitness may have some positive impact on salivary secretion in children and adolescents due to improved autonomic regulation. Considering the lack of differences in VO2max between groups after returning from school vacations (a long detraining period), no differences in SIgA secretion were expected. However, although during the 8 weeks of volleyball training, VO2max significantly increased in the competitive group, this was not accompanied by changes in salivary secretion. A recent systematic review concluded that physical training may have little impact on autonomic regulation in healthy children54 and may not account for changes in salivary secretion. Therefore, the improved aerobic capacity may not reflect a positive impact on parasympathetic regulation of salivary secretion.
Previous studies have suggested that increased BMI may account for increased levels of alpha-amylase and decreased salivary SIgA in healthy children.16,17 Increased BMI is associated with low cardiorespiratory fitness and poor autonomic regulation, although these effects can be overcome by physical training in adult individuals.55 In the present study, BMI was not correlated with alpha amylase activity, suggesting other factors may regulate saliva secretion in this study population. Salivary secretion of alpha-amylase is modulated by parasympathetic–sympathetic outflow on salivary glands and is considered a marker of sympathetic modulation,56,57 which could potentially be modulated by training status and fitness.12,15 Contrary to our hypothesis, no correlation was found between fitness and improvement in performance with secretion of salivary alpha-amylase at rest. In older men, salivary alpha amylase response is similar in low and highly physically active subjects, suggesting no differences in this salivary biomarker at rest and without stressful stimulus.58 In the same way, it is possible that no difference in alpha amylase activity was detected in the present study because the girls were not exposed to a stressful challenge during saliva sampling and might not have upregulated sympathetic outflow under training adaptation.
We concluded that despite improving physical fitness, 8 weeks of recreational volleyball training is not able to improve salivary SIgA secretion or alter alpha-amylase activity in female children and adolescents. In the studied population, physical fitness and the caries index were not related to SIgA and alpha-amylase levels.
Conflict of interestThe authors declare that they don’t have any conflict of interest.
The authors would like to thank the Coordination for the Improvement of Higher Education Personnel (CAPES) for granting a scholarship to C.P.S. (grant number 06172286986/2015) and the Fundação Araucária – PR for granting a scholarship to R.G.P. (174/2015). We thank the National Council for Research and Development (CNPq) for granting funding for the development of the study (grant no. 482524/2013-8). We thank the Secretary of Sports of the Municipality of Cambé, Paraná, Brazil and the coordinating teachers of School Volleyball, Reginaldo Mazzola and Eduardo Fiel.