Histoarchitectural arrangement of muscle is prolonged with tendons, establishing a relationship through myotendinous junctions. However, the characteristics of the transition between myoconnective structures and tendon are not well known and it remains unclear whether the histoarchitectural organization of the extracellular matrix (ECM) of both organs continues to maintain the same structuring.
The connective framework or muscle extracellular matrixThis connective framework, muscle ECM or intramuscular connective tissue (IMCT) determines the spatial organization of the skeletal muscle, i.e. The structural support of the organ. The IMTC is basically divided into three layers: epimysium (which completely surrounds the muscle externally), perimysium (which surrounds or groups muscle fibres into bundles) and endomysium (which surrounds each individual muscle cell or fibre, the structural and functional unit of the skeletal muscle). These three levels of IMTC organisation generally differ in composition and structure.1 There is a final layer, the basal lamina, which surround each muscle fibre and which is really a differentiation of the endomysium,2 due to which, with regards to structural effects, we have included both under the single term of endomysium.
Purslow3 shows that it is inappropriate to say that the endomysium and perimysium isolate or separate fibres and muscle fascicles as independent elements since what they really create is an interconnected three-dimensional network (also to the epimysium). This is perfectly in keeping with the fact that the transmission of contraction force of the fibres not only occurs at tendon level, but also through lateral transmission between neighbouring muscle fibres and fascicles within a muscle.4,5
The IMTC is not limited to this organisation but presents with a special structural configuration in the points where the muscle joins up with the aponeurosis and the tendon.6
Another important term is the fascia. This is applied to a fibrous connective tissue sheath which envelops a muscle or a set of muscles, establishing myofascial continuity between the different muscles of the extremity, which facilitates total integration of the functional activity of the muscle fibres.7 Only when this layer of connective tissue is well defined can it be called a fascia.8 Today, the term fascia has been replaced by the much wider term of fascial system.9,10 From a histological viewpoint there are differences between the fasciae since whilst some are made of loose or areolar connective tissue, others are made of thick connective tissue.7 It is the fascia which is called deep that envelops all the muscles of the body, although with variations depending on the region and on several well-defined histological traits.8 The function of this fascia is to convey strength in any direction thanks to the different orientations of its collagen fibres.11 In the case of the skeletal muscle the deep sheath surrounds the epimysium separated by loose connective tissue which allows for the free sliding of the muscle.8
The connective tendinous frameworkFrom a hierarchical point of view, the tendon displays a similar histoarchitectural organisation to the skeletal muscle. However, there is no clear terminological or structural equivalence to the IMTC.
The tendon is thus externally covered throughout its length by the epitenon, a layer of loose connective tissue which acts as a smooth sliding surface for the tendinous fascicles and is also the source of nervous and lymphatic vascular systems supply for the tendon. Inside it the endotenon (which is the basement membrane with IV and VI collagen) surrounds each tendon fibre and also links individual fibres into larger units of fibres building up the tendinous fascicle. Towards its exterior the endotenon continues with the epitenon.
Another layer which several tendons may have is the paratenon, a synovial sheath that surrounds the epitenon and from which both together receive the name of peritenon.12 The paratenon function is to provide lubrication to help cushion the tendon and reduce the friction of the adjacent tissues as the tendon extends and relaxes.13
The tendons are also externally enveloped by fasciae. Apart from surrounding the muscle, the deep fascia surrounds the tendons forming a medium around both.7 In our opinion this means that paratenon and sheath could be the same structure: both are observed in ultrasound as a hyperechoic line8,14 and where this line presents one of them, the other is absent.14 The difference would revolve around the fact that whilst the paratenon displays synovial sheathing between it and the epitenon, the sheath would be separated from the epitenon by a loose connective tissue (in the same way as between sheath and epimysium).
Continuity of connective musculotendinous frameworksThe tendon is connected to the muscle by the myotendinous junction and continues through the walls and fascia towards the muscle.15 One of the outstanding characteristics both in the tendon and the muscle is that the collagen fibres, which constitute the main structural support elements of both organs tend to present a high degree of alignment16 and this suggests a logical continuity between both connective structures. In any event, the organisation and the orientation of the collagen fibres is much more complex in both muscle17,18 and tendon.13
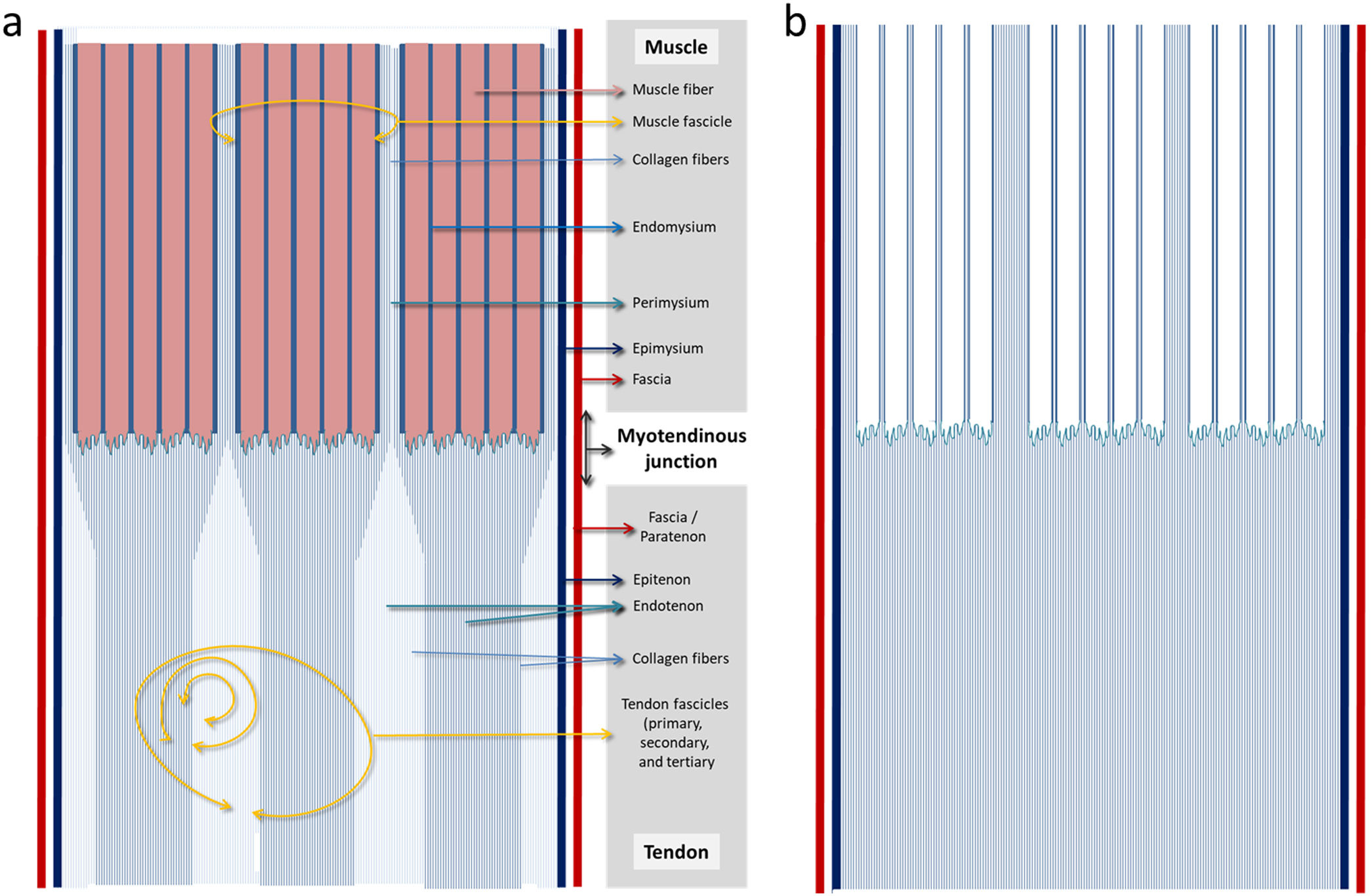
The epimysium continues with the epitenonAt the muscle ends, the epimysium thickens and fuses with the tendons.7,19 This relationship of continuity suggests that the epimysium acts like a surface tendon to help convey force from the muscle fibre to the skeleton (since the perimysium is connected to the epimysium).20 The fascia surrounding the epimysium therefore would continue with the fascia or paratenon (if applicable) which envelops the epitenon (Fig. 1).
Diagram of the structural continuity between muscle and tendon. (a) The muscle fascia continues with the tensinous fascia (or paratenon) and the epimysium with the epitenon. We have highlighted how the endomysium ends right at the muscle fibres and the perimysium at the end of the fascicle continues penetrating in the tendon and forming part of it. (b) The muscle fibres have been removed and the highlighting of the endomysium and perimysium threfore show the continuity of the musculotendinous connective frameworks with greater clarity.
The perimysium directly continues with tendon. One of the structural organizations of the perimysium in muscle is the honeycomb tubing which connects the two tendons; the ends of these perimyseal tubes form tendons and aponeuroses.20 In this way the collagen fibres of the perimysium directly penetrate the tendon, and become part of it.21
This implies that when we specifically refer to the myotendinous junction region or myoaponeurotic junction (the specific point where the muscle fibre ends are anchored onto the collagen fibres) we would really be in perimysial territory. Not, however, in the one which surrounds or groups a set of muscle fibres, but rather in the terminal end (more or less fusiform) of the muscle fascicle where the perimysial collagen fibres penetrate in the tendinous regions (Fig. 1).
The endomysium would not have continuity with the endotenonBased on the above, there would be no continuity between the endomysium and the endotenon. The endomysium would fuse with the terminal ends of the perimysial tubes (Fig. 1).
Although both the endomysium and endotenon mostly contain type IV and type VI collagen,12 as structural components a continuity is not maintained between them. Whilst the endomysium refers to the connective tissue which individually surrounds each muscle fibre,21 the endotenon delimits the tendinous fascicle.13 The tendinous fascicle (functional tendon unit), made up of a bundle of collagen fibres is delineated by the endotenon and this in turn means it can be classified into primary, secondary and tertiary fascicles13 (Kannus, 2000) easing intratendinous slippage during load.22
ConclusionsAlthough the above may be considered in general, it is probable that this pattern may vary depending on the muscle and its relationship with the tendon or aponeurosis. The characteristics of structural continuity between muscle and tendon are an important component of muscle physiology. Here it is important to remember that both the amount of IMTC and its morphological distribution is highly variable between muscles with different functions3,19 just as variability is important in form, size and location (intra or extramuscular) of tendons.23,24
This variability may also complicate or hinder the diagnosis of lesions which occur in these regions in a sports environment. In a muscular lesion a myoconnective junction is always involved, whether this be at myotendinous or myofascial level.25 Due to this, the characterisation and systematisation of the different histoarchitectural patterns in the muscle-tendon relationship is essential for classifying26 or being familiar with the clinical characteristics, correct diagnosis, prognosis and physiotherapy for the different lesions.25,27